
- Treatment of adult patients BRAF V600+ unresectable Stage III or metastatic (Stage IV) melanoma

- Adjuvant treatment of adult patients with BRAF V600+ melanoma and involvement of the lymph node(s), following complete resection

aProspective study of 134 patients with Stage IIIA–C melanoma who underwent therapeutic lymph node dissection without neoadjuvant therapy at the Princess Alexandra Hospital Melanoma Unit. DNA isolated from lymph node tumours was screened for 46 mutations in 26 genes using the extended Sequenom MelaCARTA panel. Mutations in BRAF were detected in 57/124 (45%) patients. Median duration of follow-up for survivors was 30.0 months (range: 1–141 months). The study endpoint was recurrence-free survival.4
bMeta-analysis of 52 studies with a pooled population of 7519 patients with malignant melanoma (Stages I–IV). The aim of the study was to assess the impact of BRAF mutations versus BRAF WT melanomas on OS, PFS and DFS.6
cCOMBI-d: Phase 3 trial, 423 patients with unresectable stage IIIC or IV melanoma (BRAF V600E/K mutations) were randomly assigned to receive TAFINLAR (150 mg twice daily) plus MEKINIST (2 mg daily) or TAFINLAR plus placebo. The primary endpoint was PFS, with secondary endpoints of OS, RR, DOR, and safety. An interim OS analysis was preplanned. For OS a specified efficacy-stopping boundary (two-sided p=0.00028) was not crossed.10
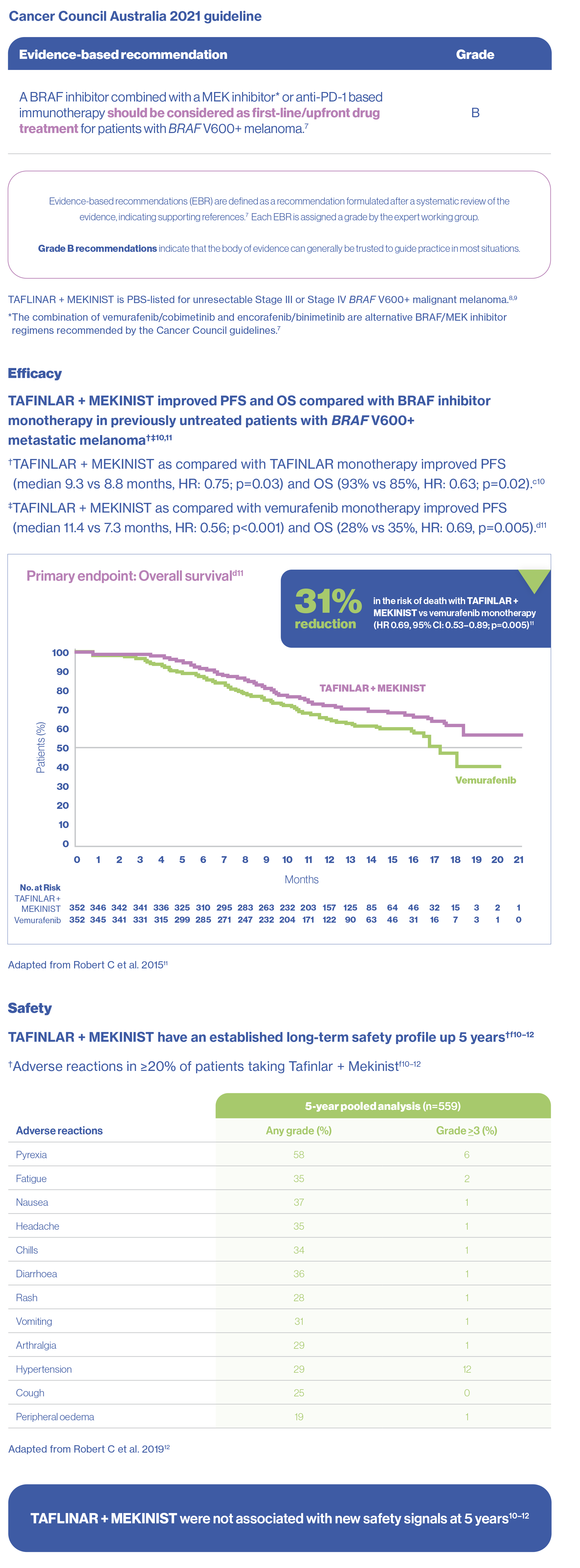
dCOMBI-v:Phase 3, multicentre, open-label, randomised (1:1), active-controlled trial of 704 patients with unresectable (stage IIIC) or metastatic (stage IV) BRAF V600E/K mutation-positive cutaneous melanoma. Patients were randomised to receive TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily (n=352) or single-agent vemurafenib 960 mg twice daily (n=352). Treatment was continued until disease progression or unacceptable toxicity. The primary endpoint was OS. Secondary endpoints included PFS, ORR, DOR and safety.11
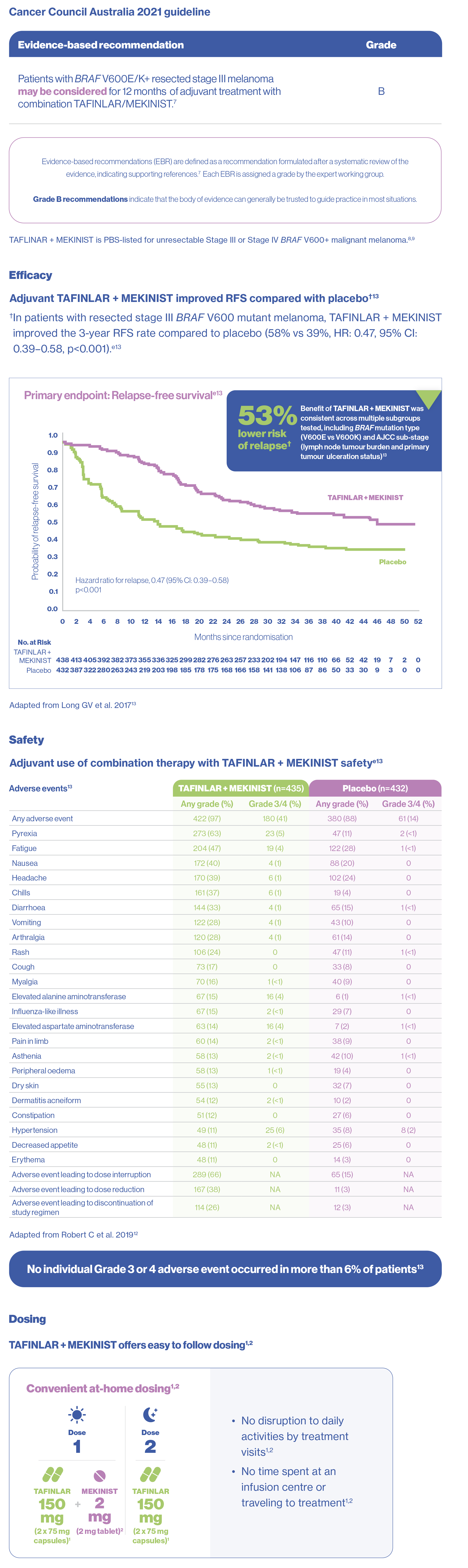
eCOMBI-AD:Phase 3 placebo-controlled trial, 870 patients with fully resected stage III melanoma (BRAF V600E/K mutations) were randomly assigned to receive TAFINLAR (150 mg twice daily) plus MEKINIST (2 mg daily) (438 patients) or placebo (432 patients) for 12 months. The primary endpoint was relapse-free survival.13
fCOMBI-d/v:Pooled analysis of COMBI-d and COMBI-v consisted of 563 first-line patients with BRAF V600E/K mutation-positive unresectable or metastatic melanoma. All patients in the analysis received TAFINLAR 150 mg twice daily + MEKINIST 2 mg once daily in either COMBI-d (data cutoff, December 10, 2018) or COMBI-v (data cutoff, October 8, 2018). Median follow-up was 22 months (range, 0–76).12
AE, adverse event; AJCC, American Joint Committee on Cancer; CI, confidence interval; BRAF, B-raf proto-oncogene; DFS, disease-free survival; DOR, duration of response; HR, hazard ratio; MEK, mitogen-activated protein kinase; ORR, objective response rate; OS, overall survival; PBS, Pharmaceutical Benefits Scheme; PD-1, programmed death-1; PFS, progression-free survival; RFS, relapse-free survival; RR, response rate; WT, wild type.
REFERENCES: 1. TAFINLAR (dabrafenib) Australian approved product information. 2. MEKINIST (trametinib) Australian approved product information. 3. Turski ML et al. Mol Cancer Ther 2016; 15(4): 533–547. 4. Barbour AP et al. Eur J Cancer 2014; 50: 2668–2676. 5. Long GV et al. J Clin Oncol 2011; 29(10): 1239–46. 6. Ny L et al. Acta Oncol 2020; 59(7): 833–44. 7. Cancer Council Australia. Clinical practice guidelines for the diagnosis and management of melanoma. (2021). 8. Pharmaceutical Benefits Scheme. TAFINLAR (dabrafenib). (2024). 9. Pharmaceutical Benefits Scheme. MEKINIST (trametinib). (2024). 10. Long GV et al. NEJM 2014; 371(20): 1877–88. 11. Robert C et al. NEJM 2015; 372(1): 30–9. 12. Robert C et al. NEJM 2019; 381(7): 626–36. Supplementary appendix. 13. Long GV et al. NEJM 2017; 377(19): 1813–23.
MEKINIST and TAFINLAR PBS Information: Authority Required (STREAMLINED). Please refer to PBS Schedule for full authority information for products.
 MEKINIST and TAFINLAR are subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
MEKINIST and TAFINLAR are subject to additional monitoring in Australia. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse events at www.tga.gov.au/reporting-problems.
For MEKINIST prescribing information, please click here.
For TAFINLAR prescribing information, please click here.
This content is intended for Australian healthcare professionals and is promotional.

